This is the course blog for Semester 1, 2011 at the University of Queensland
3/31/11
1st Brillouin Zone
Everything within the lattice may be described as something within the 1st Brillouin zone of the reciprocal lattice, displaced by a vector G. This is how we get the "reflections" in the reduced zone of the energy- k plots seen in the notes and lecture.
3/30/11
Assumption of Weak Potential
There are a number of instances where the assumption of a weak potential gives an accurate description of electrons moving through a lattice, that is, the electrons behave almost as free electrons. There are two reasons why this is sometimes the case.
Firstly, the interaction of electrons with the ions is much stronger at small separations, the conduction electrons are separated from the ions by the core electrons that surround it, and so the interaction is weak. Secondly, in this region in which the conduction electrons are allowed, their mobility can have the effect of screening the fields of the ions, making the potential experienced by any given conduction electron very weak.
Firstly, the interaction of electrons with the ions is much stronger at small separations, the conduction electrons are separated from the ions by the core electrons that surround it, and so the interaction is weak. Secondly, in this region in which the conduction electrons are allowed, their mobility can have the effect of screening the fields of the ions, making the potential experienced by any given conduction electron very weak.
Photonic Crystals
Recently we covered Bloch's theorem and its application to the motion of electrons in a periodic potential, i.e. our crystal lattice of ions. The theorem however is not strictly contained to crystal structures, as it only discussed the translationally invariant Hamiltonians and the resulting form of wavefunctions.
An electromagnetic analogue of the above behaviour is found in optical nanostructures which exhibit a periodic potential, due to a periodic dielectric of the material. The behaviour of the EM field in the structure is found to mirror that of the electrons in the crystal structure.
Although we haven't yet covered it, the greatest similarity is between the Bloch treatment of semi-conductors and this photonic crystal. Analogous to the semi-conductor, forbidden energy zones or wavelength band gaps are formed. This gives rise to a range of behaviours such as inhibition of spontaneous emission and and high reflecting omni-directional mirrors.
An electromagnetic analogue of the above behaviour is found in optical nanostructures which exhibit a periodic potential, due to a periodic dielectric of the material. The behaviour of the EM field in the structure is found to mirror that of the electrons in the crystal structure.
Although we haven't yet covered it, the greatest similarity is between the Bloch treatment of semi-conductors and this photonic crystal. Analogous to the semi-conductor, forbidden energy zones or wavelength band gaps are formed. This gives rise to a range of behaviours such as inhibition of spontaneous emission and and high reflecting omni-directional mirrors.
3/29/11
Tutorial: Week 5
During tomorrow's tutorial we're going to look at some of the questions from "Tutorial 3: Crystal Structures and the Reciprocal lattice". To get the most benefit out of the tutorial please have a look at the questions beforehand if you have the chance.
Assignment 3: due Monday April 11
Trying to Understand Bloch's Theorem "Oxford Dictionary"
A theorem relating to the quantum mechanics of crystals stating that the wave function ψ for an electron in a periodic potential has the form ψ(r) = exp(ik · r)U(r), where k is the wave vector, r is a position vector, and U(r) is a periodic function that satisfies U(r+R) = U(r), for all vectors R of the Bravais lattice of the crystal. Bloch's theorem is interpreted to mean that the wave function for an electron in a periodic potential is a plane wave modulated by a periodic function. This explains why a free-electron model has some success in describing the properties of certain metals although it is inadequate to give a quantitative description of the properties of most metals. Bloch's theorem was formulated by the German-born US physicist Felix Bloch (1905–83) in 1928.
3/28/11
Sommerfeld model's assumption
In this model free electron model is modified by tacking into account quantum statistics and the Pauli exclusion principle.
- Free electrons are valence electrons of composing atoms
- A valence electron in metal finds itself in the field of all ions and that of other electrons. The mutual repulsion between the electrons is neglected and the potential filed representing the attractive interaction of ions is assumed to be completely uniform every where inside the solid.
- Distribution of energy in an electron gas obeys the Fermi-Dirac quantum statistics.
- Only electrons close to the Fermi energy are scattered.
The energy levels are filled in accordance with the Pauli’s exclusion principle according to which an energy level can accommodate at the most two electrons, one with spin up and one with spin down.
- Free electrons are valence electrons of composing atoms
- A valence electron in metal finds itself in the field of all ions and that of other electrons. The mutual repulsion between the electrons is neglected and the potential filed representing the attractive interaction of ions is assumed to be completely uniform every where inside the solid.
- Distribution of energy in an electron gas obeys the Fermi-Dirac quantum statistics.
- Only electrons close to the Fermi energy are scattered.
The energy levels are filled in accordance with the Pauli’s exclusion principle according to which an energy level can accommodate at the most two electrons, one with spin up and one with spin down.
3/27/11
Structure factor
A structure factor give a description of how the incident radiation scattered by a material. It is given by summing the scattering from all the atoms of the unit cell. it is important because it gives an ability to interpret interference patterns that can be obtained in X-ray, electron and neutron diffraction.
Useful animation helps to understand how to determine position of an atom in a cubic
I used to have problem with determination of atom in a cubic. I found animation really useful and hope it will help. http://www.matter.org.uk/diffraction/intensity/structure_factor_bcc.htm
Definition of unit cell and primitive cell
A unit cell is the group of atoms that has all the properties and symmetries of the whole lattice and we can make the whole structure of lattice by repeating that.
we can define the crystal structure by a smallest unit cell. This cell is called the primitive unit cell which its shape is not unique but its area is.
Summary of Bragg diffraction
Bragg peak is the peaks of scattered radiation which is the constructive interference of reflected X ray. It occurs at specific combination of wavelengths and angle of incidence. The ideal scattering is the one that only the direction is changing not the wavelength
3/26/11
crystal structure
I found an amazing website talking about most of the topics that we have studied in classes .This web written by Department of Crystallography and Structure Biology and associated with lots of graphs and animations . I hope it would be useful .
3/25/11
crystal systems are seven or six ?
I read one interesting article about crystal systems that are six in the United State schools, while they are seven on other countries. The reason for this in this link http://www.yourgemologist.com/crystalsystems.html and you will see amazing pictures about seven crystal system .
The crystal system

I found that to classify the crystal , the point groups play an important role to divide the crystal systems into 7. Also, decreasing symmetry in crystal is one of the elements to put the seven crystal system in this way and these are Isometric System, Hexagonal System, Tetragonal System, Trigonal System, Orthorhombic System, Monoclinic System and Triclinic System.
Bravis lattices
 In two dimensions, there are five distinct Bravais lattices, while in three dimensions there are fourteen. The lattices in two dimensions are the square lattice, the rectangular lattice, the centered rectangular lattice, the hexagonal lattice and the oblique lattice as shown in Figure. All the lattice points of the rectangular lattice can be obtained by a combination of the lattice vectors a1 and a2. The centered rectangular lattice can be constructed in two ways. It can be obtained by starting with the same lattice vectors as those of the rectangular lattice and then adding an additional atom at the center of each rectangle in the lattice.The lattice vectors a1 and a2 generate
In two dimensions, there are five distinct Bravais lattices, while in three dimensions there are fourteen. The lattices in two dimensions are the square lattice, the rectangular lattice, the centered rectangular lattice, the hexagonal lattice and the oblique lattice as shown in Figure. All the lattice points of the rectangular lattice can be obtained by a combination of the lattice vectors a1 and a2. The centered rectangular lattice can be constructed in two ways. It can be obtained by starting with the same lattice vectors as those of the rectangular lattice and then adding an additional atom at the center of each rectangle in the lattice.The lattice vectors a1 and a2 generateFigure[The five Bravais lattices of two-dimensi
onal crystals: (a) square, (b) rectangular, (c)
centered rectangular, (d) hexagonal and
(e) oblique )].
the traditional unit cell and the center is obtained by attaching two lattice points to every lattice point of the traditional unit cell.
Postscript Files in SSS
I'm having trouble saving the graphs as postscripts, I've tried .eps and .ps and GIMP is unable to open either of them. Does anyone know how to save them in such a way that I can edit them?
3/24/11
Bragg Formulation and von Laue Formulation
Both of these approaches explain the reflected x-ray from a lattice, so the structure of the lattice can be predicted. The Bragg Formulation has two main assumptions: 1. The x-ray will reflects "perfectly" by ions in only one lattice plane. 2. The reflected beams interfere constructively always. The von Laue Formulation is considered more sophisticated because it can explain the reflection of the rays without the need of planes grouping. But it does agree with Bragg in the second assumption.
Symmetry Operation

An interesting part about operations for lattices rotations is presented in Kittel ch1. The small graph shows 6 operations where each one rotates the lattice by 2π/n, where n=1,2,3,4,5 or 6 for the six samples in the graph. All of them should be applied to a lattice point. For the operations 1 and 2 there are no restrictions on the rotation, unlike the rest. Operations 3 and 6 require a hexagonal lattice, but operation 4 requires a square one.
How to do better on assignments and exams
Read this and put it into practice!
The difference between unit cell and primitive cell
Since the beginning of the crystal structure lectures, I have had difficulties to differentiate between the definition of unit cell and primitive lattice . However , I understand that unit cell is the smallest structure of the lattice which contains more than one lattice points and when it repeated over and over again it will build the lattice while the primitive cell is smaller than cell unit and contains no more than one point lattice
3/23/11
Lecture notes - last update!
There were some problems with a few of the images in the last version I uploaded and this one includes the extra slides I used in the lecture. Get it here.
Normal LaNi5
Neutron Scattering
In the lectures we have talked about X-ray diffraction a great deal, but the use of neutrons as probes of lattice structure was also mentioned. Neutron diffraction is used where X-rays are unable to give an accurate picture of the location of atoms. X-rays are usually scattered by the electron cloud, and more strongly for atoms with more electrons, hence, for atoms with relatively few electrons, the X-rays will not strongly interact and so the amount of scattering will be less. Neutrons are scattered by the atomic nucleus, as they do not interact with the electron cloud, and the intensity of the diffraction peaks is different for different isotopes.
3/22/11
Scattering due to atoms in a crystal
For those interested I came across a more in-depth derivation of the Von Laue condition and the structure factor.
physics.valpo.edu/courses/p440/Diffraction_Crystal_Structure.ppt
By treating the incident waves as plane waves and the atoms as point scatterers, the scattered waves take the form of isotropic spherical waves. One can then work through and derive the total scattered wave as a sum of the contributions of all the atoms.
By calculating the related intensity of this scattered wave the Laude condition falls out trivially. The derivation up to this point is slightly more complicated then that done in class, however it is mathematically more appealing and concrete.
To derive the structure factor the amplitude of the scattered wave is considered, and the the structure factor Sk is defined from it. Its nice to see how this measure can fall out from a relatively quick derivation.
physics.valpo.edu/courses/p440/Diffraction_Crystal_Structure.ppt
By treating the incident waves as plane waves and the atoms as point scatterers, the scattered waves take the form of isotropic spherical waves. One can then work through and derive the total scattered wave as a sum of the contributions of all the atoms.
By calculating the related intensity of this scattered wave the Laude condition falls out trivially. The derivation up to this point is slightly more complicated then that done in class, however it is mathematically more appealing and concrete.
To derive the structure factor the amplitude of the scattered wave is considered, and the the structure factor Sk is defined from it. Its nice to see how this measure can fall out from a relatively quick derivation.
X-ray Diffraction: here's one I prepared earlier
Tutorial week 4
As was requested last week, tomorrows tutorial will be based on Solid State Simulations. Please make sure you bring a laptop to the tutorial with a working version of the software. Trying some of the Assignment questions beforehand would be a good idea too.
3/21/11
3/20/11
Important complication for XRD
One problem with using Bragg's law to determine the structure of a material is that orienting some crystals may result in different scattering angles. This would make the structure of the material appear different to what it actually is. Defects and impurities, in great enough concentrations, may also make this problem worse.
Result of important example of reciprocal lattices
A simple cubic lattice, a body centered cubic lattice, a face centered cubic lattice and a simple hexagonal lattice have a simple cubic lattice, a face centered cubic lattice, a body centered cubic lattice and another simple hexagonal lattice respectively as its reciprocal lattice. An if the volume of a primitive cell in the direct lattice is v, the volume of the primitive cell in the reciprocal lattice is 〖(2π)〗^3/v
X-ray crystallography
X-rays can be used to determine the structure of a material. This is an application of Bragg's law where x-rays are scattered off a sample into a collector over a range of angles. Certain angles will have more x-rays scatter into the collector than others. Those angles with the highest counts of x-rays will correspond with the Miller indicies of the material.
3/19/11
The reciprocal lattice and wave diffraction
I found a useful paper on the internet talking about wave diffraction and the Reciprocal lattice . The writer gives a definition of reciprocal lattice with examples. Also , he mentions diffraction of waves by crystals .find more related to this by clicking on this link
http://phy.ntnu.edu.tw/~changmc/Teach/SS/SS_note/chap02.pdf
Epitaxial Growth Process
Epitaxial Growth is a basic tool to fabricate most modern devices. In epitaxial growth, a layer of one material is grown on a crystal of the same material or a different one. In that case, building pure layers without defects depends on the lattice constant matching.
Coordination number of face centered cubic lattice
Because I have difficulties to imagine the coordination number of face centered cubic lattice I thought this shape could help. FCC has coordination number 12 which is the number of nearest neighbors of each point. ( Fig. reference: Crystallography website)
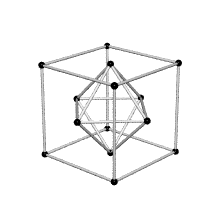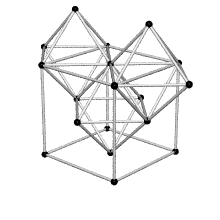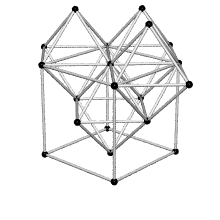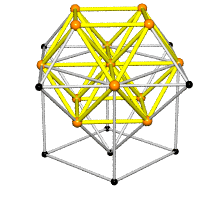

3/18/11
Crystals structure by x-ray diffraction

One of the fundamental methods in determining the structure of crystals is the x-ray diffraction. The x-ray magnitude is proportional to the spacing between atoms in crystals (1A) which makes x-ray the preferred crystallography tool.
In a perfect diffraction the incident angle equals the angel of the diffraction, and the distance between two diffractions can be determined by Bragg's law:
2 d sin ϴ = n λ
Different crystals have different densities of electrons, consequently, the scattering of the x-ray beam will differ as the beam diffracts by the planes in the crystal. A three-dimensional picture of the density of electrons within the crystal can be made by the angles and directions of the scattered beams. But as the crystal structure becomes more complex, the picture that is given by the x-ray diffraction becomes less clear. However, it has worked for crystals with hundreds of thousands of atoms. Besides, x -ray diffraction has been used to determine the structure of over 99% of the Cambridge Structural Database, which contains over 500,000 structures. So, although the basics of this method are quite simple, it is still the master method in studying crystal structures. (Fig. ref=http://www.chem.ufl.edu/~itl/2045_s99/lectures/lec_h.html)
Bravais Lattice Symmetries
I was interested in the lecture when Paul mentioned the 4th miller index being related to the symmetry of a lattice, so I looked at Chapter 7 of Ashcroft and Mermin, which talks about symmetries of a Bravais lattice.
Bravais lattices can be classified by the symmetries that their lattices exhibit. Since they are all periodic, any translational operation will result in the same lattice. In general, the bravais lattice can be classified by considering all rotations, inversions and reflections that preserve the lattice structure, i.e. it looks the same after the operation. This set of operations is called the symmetry group of the Bravais lattice.
Bravais lattices can be classified by the symmetries that their lattices exhibit. Since they are all periodic, any translational operation will result in the same lattice. In general, the bravais lattice can be classified by considering all rotations, inversions and reflections that preserve the lattice structure, i.e. it looks the same after the operation. This set of operations is called the symmetry group of the Bravais lattice.
3/17/11
LATTICE PLANES
I found some useful and interesting theorem in Ashcroft and Mermin. "For any family of lattice planes separated by a distance d,there are reciprocal lattice vectors perpendicular to the planes, the shortest of which has the length of 2pi/d. And conversely,for any reciprocal lattice vector K, there is a family of lattice planes normal to K and separated by a distance d,where 2pi/d is the length of the shortest reciprocal lattice vector parallel to K".
Family of lattice planes means a set of parallel equally spaced lattice planes, which together contains all the points of the three dimensional bravis lattice.
Family of lattice planes means a set of parallel equally spaced lattice planes, which together contains all the points of the three dimensional bravis lattice.
Solving Schrodinger with periodic boundary conditions
One of the assignment questions was to solve Schrodingers equation for a free particle with PERIODIC boundary conditions. Almost everyone solved it for a particle in a box. This has different boundary conditions.
See Ashcroft and Mermin, page 33-34 for the correct solution.
See Ashcroft and Mermin, page 33-34 for the correct solution.
crystal sturcture and unit cell
The crystal structure is a specific arrangement of atoms, while when a crystal has many simplest repeating unit this called a unit cell .As we know in cubic ,there are three type of unit cells.
A simple cubic unit cell (SC) that contains eight identical particles on the eight corner,the body centered cubic unit cell (BCC) that contains nine identical particles(one on the centre and eight on the corner) and the face centered cubic unit cell (FCC) that contains 14 identical particles (6 faces and 8 on the corner).
A simple cubic unit cell (SC) that contains eight identical particles on the eight corner,the body centered cubic unit cell (BCC) that contains nine identical particles(one on the centre and eight on the corner) and the face centered cubic unit cell (FCC) that contains 14 identical particles (6 faces and 8 on the corner).

Peltier Effect
Having discussed the Seebeck effect in the tutorial, its interesting to note that there also exists a similar effect when one applies a voltage to a coupling of two metals. The applied potential creates a temperature gradient between the metals, the reverse of the Seebeck effect. The equation governing the effect is:
dQ/dt = (pi_A - p_B)I
Where here dQ/dt is the "Peltier heat" absorbed per unit time, I is the current and pi-A, pi-B are the Peltier coefficients of each material. An interesting device which makes use of this effect is the Peltier cooler. http://en.wikipedia.org/wiki/Thermoelectric_cooling
3/16/11
Close Packing and Packing Fraction
I was interested in the various close-packing arrangements possible, in class we talked about hexagonal close packing (hcp), in which each layer is equivalent to (directly above) the layer 2 positions below it. One way to characterise the packing of atoms in a crystal structure is the 'Packing Fraction', which is the % of Volume of the unit cell taken up by atoms, i.e. Fraction = N*V_a/V_c, where N is the number of atoms in a unit cell (can be non-integer), V_a is the volume of an atom and V_c is the volume of the unit cell. The body-centred cubic bravais lattice has a packing fraction of 0.68, while the hcp has a fraction of 0.74.
Crystal Structure and Pure Maths
In the lectures it was mentioned how one can mathematically define a structure to be a Bravais lattice if it admits a representation R = n1a1 + n2a2 + n3a3. Due to the periodic nature of crystal structures, I was curious if anyone has come across research in this field taken from a purely mathematical approach. It seems to me that there could be some interesting ideas in terms of crystal structure if one was to apply group theory and abstract algebra to the structures.
I'm sure it would have been done, however I was just interested if it was still a fairly active research area.
I'm sure it would have been done, however I was just interested if it was still a fairly active research area.
3/15/11
one failed aspect of Sommerfeld model
Sommerfeld model has failed to explain the mean free path (l) which equal 100 angstrom.The electron seams to pass this distance in crystal passing thousands of charged ions without scattering . As a result ,we will find the solution of this puzzle in Bloch model.
Assignment 2: due monday March 28
Tutorial week 3
Before tomorrow's tutorial (wed. 2pm) try and do question 2 on tute 1 and question 3 on tute 2.
Towards understanding crystal structures
There is a really nice non-mathematical chapter on Crystal structures and symmetry in the beautiful book The Material World by Cotterill. You can download a scan of the chapter from the first edition here.
3/14/11
A problem with SSS
I couldn’t open my saved graphs with solid state simulations. I did have .pre as an extinction but couldn’t open them.
Did someone do it?
Please can u tell
Did someone do it?
Please can u tell
3/13/11
Crystal structures notes
You can download a copy of the slides I will be using this week to tell you about crystal structures here.
Drude prediction of tranparency of metals to ultraviolet light
Did we cover this in the lectures? I don't really remember.
It is in Ashcroft and Mermin and also in some notes of mine from the 3rd yr SS course I did at Griffith.
The Drude model explains how visible and other low frequency light are reflected off the surface of metals yet to light above a certain frequency, metals become very transparent.
This is a consequence of the drude model's AC conductivity in metals when an external electric field is applied which is dependent on the frequency of electric field.
When the frequency of the electric field is much greater than the relaxation time^-1, the real part of the conductivity (largely) disappears and the solutions for k in the wave equation become real. This allows the high frequency (usually ultraviolet) light to propagate through metals.
For lower frequencies, the solutions for k in the wave equation are imaginary resulting in an exponential decay of the electric field as it travels into the metal.
The transition frequency between these two cases is known as the plasma frequency.
It is in Ashcroft and Mermin and also in some notes of mine from the 3rd yr SS course I did at Griffith.
The Drude model explains how visible and other low frequency light are reflected off the surface of metals yet to light above a certain frequency, metals become very transparent.
This is a consequence of the drude model's AC conductivity in metals when an external electric field is applied which is dependent on the frequency of electric field.
When the frequency of the electric field is much greater than the relaxation time^-1, the real part of the conductivity (largely) disappears and the solutions for k in the wave equation become real. This allows the high frequency (usually ultraviolet) light to propagate through metals.
For lower frequencies, the solutions for k in the wave equation are imaginary resulting in an exponential decay of the electric field as it travels into the metal.
The transition frequency between these two cases is known as the plasma frequency.
3/12/11
problem with the Drude program in Solid state simulation
I have tried to open the Drude program on my computer which is macbook pro but I could not
because their are many files inside it so I do not know which one I have to choose to open the program , if any one know about that please tell me how
thanks
Sommerfeld and Fermi-Dirac distribution
The Drude model assumed that there is no Pauli exclusion principle, but in Sommerfeld model we have two electron with spin up and down in each energy state. Electrons occupy the states up to the Fermi energy, but only electrons close to the Fermi energy can move and scatter and electrons below the Fermi energy must stay still. That is the consequence of Pauli exclusion principle.
3/11/11
Density of States
I have found the Sommerfield model to be quite a more enjoyable theory to examine in contrast to the Drude model. Even though the Drude model was based on simple kinematics, the Sommerfield model appears to me to be a more justified approach to the behaviour of conductors. Perhaps this is because of my previous experience in statistical mechanics and that I am comfortable with such quantities as the density of states.
What do other people think? Also for those who have done phys3020, did you prefer the approach we took or that of the text by Ashcroft in deriving and justifying the density of states?
Summary of Sommerfeld Theory
I am just trying to state some of the outcomes and drawbacks of the Sommerfeld theory. The Sommerfeld theory was useful to get the solution for the Schrodinger equation to get the allowed electronic states, Fermi-Dirac distribution was used to determine the population of states and the electron scattering (unknown mechanism) was also well explained. So the Sommerfeld theory was more helpful to give the better shape for the free electron model.
There were numbers of questions which were unanswered by the Sommerfled theory like; the true mechanism for the electron scattering, the unhindered motion of the conduction electrons in metal for the significant distance, the dominant contribution to the heat capacity of t he solid for T>=10K, and why only the positive charged particles are responsible for the electricity conduction in some metals?
There were numbers of questions which were unanswered by the Sommerfled theory like; the true mechanism for the electron scattering, the unhindered motion of the conduction electrons in metal for the significant distance, the dominant contribution to the heat capacity of t he solid for T>=10K, and why only the positive charged particles are responsible for the electricity conduction in some metals?
Tips on blog posts
Make sure you include a title on your post.
Don't write a comment on another post if your "comment" is actually on a different subject. Start a new post instead.
Don't write a comment on another post if your "comment" is actually on a different subject. Start a new post instead.
3/10/11
When I read the Solid State Physics chapter one I found that Drude predicted that thermal current flows opposite to the direction of the temperature gradient because his research actually based on Classical Statistical Mechanics. However, by using Quantum Mechanics his prediction would be corrected .It is right?
Drude assumed that collisions were due to electrons scattering off heavy ions. When the distance travelled between collisions (mean free path) was calculated, the result seemed to agree with that assumption, as the mean free path was approximately the same as the spacing between atoms, suggesting that they were the cause of collisions.
I found it interesting how although we now know the Drude model's predictions for the scattering time tau and the electron velocity v_0 (from equipartition theorem) to be incorrect, there is still a number of quantities that are independent of the scattering time that the Drude model can successfully predict.
I found it interesting how although we now know the Drude model's predictions for the scattering time tau and the electron velocity v_0 (from equipartition theorem) to be incorrect, there is still a number of quantities that are independent of the scattering time that the Drude model can successfully predict.
3/9/11
The Drude model fails to describe the dependence of the temperature to the magnetic susceptibility, specific heat and the value of Lorenz number. However, the Drude model give the universal value of Lorenz number but there is a difference by a factor of 2. These issues can be solved by the Sommerfeld model.
Tutorial questions
Here are some tutorial questions that we may look at during the semester. Try and do them by yourself and I will then give help as needed to do them.
Some of these questions have been exam questions in the past.
Some of these questions have been exam questions in the past.
3/7/11
Updated lecture notes
Here are the latest versions of my powerpoint slides on the Drude model and on the Sommerfeld model.
But, reading Ashcroft and Mermin, chapter 1 and 2 is much better than reading the slides, because they have all the details and fewer mistakes!
But, reading Ashcroft and Mermin, chapter 1 and 2 is much better than reading the slides, because they have all the details and fewer mistakes!
When considering the overall successes/failures of the Drude model, its quite interesting to actually take a step back and revisit the initial assumptions/calculations of the Drude model. Clearly the dominating assumption is the constraint that electrons only interact with the ions of the material, and not other electrons, giving rise to the relaxation time, tau.
It is interesting that by using this variable along with the tools of basic mechanics taught in first year physics (though perhaps we would not have found them so basic back at that time) such a range of important quantities can be estimated, let alone the fact that quite a few of these give reasonable results. It is even more interesting given that when one considers how far removed quantum mechanics is from basic mechanics when dealing with particles such as electrons. One would far more likely expect that the Drude model and its basic derivation would be completely useless in understanding or predicting any physical properties of materials. However in the tradition of approximations in physics, it serves as a sound basis to begin looking at the physics and behavior of various materials.
Where are your blog posts?
There are 40 formative points for posting on this blog.
As stated in the course profile:
To receive 3 points per week, a student must post at least two items and two comments on other student posts. Posts can include summaries of the reading (especially focusing on what is the key idea, key figure, or key equation for a section), questions the students have, or links to material related to the course.
Since no student posted last week, you all get zero for week 1.
As stated in the course profile:
To receive 3 points per week, a student must post at least two items and two comments on other student posts. Posts can include summaries of the reading (especially focusing on what is the key idea, key figure, or key equation for a section), questions the students have, or links to material related to the course.
Since no student posted last week, you all get zero for week 1.
3/1/11
Tutorial wednesday 2pm
Please bring your laptop with an installed (and hopefully working) version of solid state simulations and a copy of Assignment 1.
Drude slide update
Here are a more up to date version of the Drude model slides from todays lecture
The Drude model
Today I will begin to discuss the Drude Model, the simplest model for the electronic properties of metals. A copy of the power point slides is here. They were originally produced by Associate Professor Ben Powell, a previous lecturer in this course.
The lecture will closely follow Chapter 1 in Aschroft and Mermin.
The lecture will closely follow Chapter 1 in Aschroft and Mermin.
Subscribe to:
Posts (Atom)


